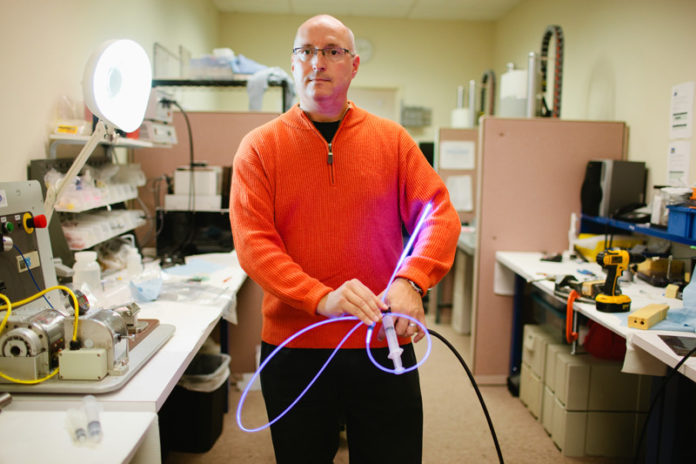
For the past year venture capitalists have kept a watchful eye on surgeries like the broken-ankle repair Dr. Thomas Gausepohl performed on a woman in Marl, Germany, in June 2011.
Confronted with a bone fracture that would normally require inserting up to 10 screws and a plate in the woman’s ankle, or immobilizing it for six weeks, Gausepohl chose a new, minimally invasive bone-stabilization technique involving balloons, glue and light fiber.
Less than 24 hours after the procedure, the woman was able to walk out of the hospital on the injured ankle without a cast or brace.
And investors had another example of the vast potential of new devices developed for the procedure by IlluminOss Medical Inc. of East Providence.
This fall, after more than 100 clinical trials in Europe and more still in South America, IlluminOss closed on a $28 million Series C funding round from a group of nine investors on both sides of the Atlantic.
The funding round was the largest for a company that was founded in Rhode Island in at least a decade and marks the business as a major candidate for life sciences growth in the state.
“It is the single largest venture-capital financing in a Rhode Island biomedical startup in a long time,” said Richard Horan, managing director of the Slater Technology Fund, which invested $500,000 in the Series C round, “But the caliber and commitment of those investors is equally worth mention. Medical devices of the type being pioneered by IlluminOss [and used by Gausepohl] are not for the faint of heart. They are best financed by investors who have the experience to carry them through to completion.”
The Series C round was co-led by new investors Tekla Capital Management of Boston and Life Sciences Partners of Amsterdam, Munich and Boston. The round also included previous IlluminOss investors Foundation Medical Partners of Connecticut, Mieza Partners of Connecticut and New Leaf Venture Partners of New York, plus new investors Excel Venture Management of Boston, SR One of Philadelphia, and Pappas Ventures of Research Triangle Park, N.C.
Horan said the last Rhode Island company he remembers raising a larger amount of funding in a single round was Cytotherapeutics Inc. of Providence in 1998.
Rabiner dreamt up the concept behind the company’s fracture-repair system when a relative went through a long and painful bone-stabilization surgery while Rabiner was investigating fly-fishing glues. He then took the idea to orthopedists at Rhode Island Hospital who advanced the concept.
Instead of using pins, screws and plates to set tough breaks, the IlluminOss system inserts a balloon, light fiber and photo-sensitive glue into the damaged bone through a catheter.
The balloon is inflated and conforms to the interior shape of the bone and when the glue is exposed to the light, it hardens in that shape, supporting the bone from the inside.
The device is deployed through a one-quarter or one-eighth of an inch hole into the bone that is much smaller than the cuts required to set bones using plates and screws.
The capital raised on the Series C will finance two parallel growth tracks for IlluminOss.
First, the company is launching its devices commercially in Europe – where it is already approved for use – which will require scaling up manufacturing, sales and marketing.
Second, the company is entering clinical trials with the U.S. Food and Drug Administration for what it hopes will be approval for sales in the United States.
IlluminOss President and CEO Scott Rader said the new financing should give the company enough capital to grow and pursue both of those tracks for three-and-a-half years.
“In Europe we have been doing a private-stage launch to prove the technology clinically and the results have been nothing short of amazing,” Rader said. “We treated well in excess of 100 patients, are currently a clinical-stage company and in the fourth quarter we will have a commercial launch in Europe.”
As device production scales up, assembly of all IlluminOss systems will take place in the company’s Waterman Avenue plant using components manufactured by several local part makers that will see an increase in business, Rader said.
Rader declined to specify how many new workers IlluminOss will add, but said hiring will take place across the organization, from manufacturing to administration and clinical research. At this point the company, which employs 15, has extra space to grow in its existing plant.
Although the IlluminOss system has been used in patients ranging in age from 17 to 103, Rader said it is particularly valuable for treating older patients whose bones have weakened through osteoporosis and often don’t hold screws.
IlluminOss has 10 American patents and five international patents, Rader said, and is the only company he knows pursuing this method of repairing bones.
“I see this becoming one of the standards of care in the stabilization and repair of bones,” Rader said. “We have patents not only on low-load-bearing bones but have extended into spine, cranial-facial and sports medicine.”
Rader, the former CEO of Arsenal Medical in Watertown, Mass., and Bacchus Vascular (which was acquired by Covidien) was hired by IlluminOss in June 2010 to lead the company into its commercialization phase.
Rabiner is now founder and chief technical officer and still intimately involved “driving his original vision of the technology,” Rader said.
Before starting IlluminOss, Rabiner founded Selva Medical, a device maker backed by Slater. Selva was bought by W.L. Gore in 2006, but, while the company moved on, Rabiner remained in the state and began working on new projects.
Slater’s Horan says the next key things to watch for at IlluminOss are the results of key studies of patient outcomes in Europe and then the sales that should follow the commercial launch.
With Rhode Island’s leaders desperate to transition the state to a knowledge-based economy, Horan said companies like IlluminOss will be the driving forces and investments like the recent Series C the key milestones.
“As the community engages in the conversation about how to accelerate the innovation economy here, it is companies like IlluminOss that will be the vanguard,” Horan said. •













[…] was founded more than a decade ago by Robert Rabiner and has attracted more than $50 million in investment since then based on the promise of its products as well as the potential size of the […]