PROVIDENCE – The R.I. Department of Health paused administration of the Johnson & Johnson vaccine this morning, following on a recommendation to states from federal health officials who have opened a review of its safety after six people developed serious blood clotting as a side effect.
The announcement, made as the state’s vaccine committee began its review, was made at about 7:30 a.m. Tuesday.
It did not affect the day’s appointment release, which was for vaccines developed by Moderna and Pfizer-BioNTech. At 9 a.m., the state released another 7,600 appointments through its central website, vaccinateri.org, for those shots. The state is now administering vaccine to all Rhode Islanders age 40 and older, as well as people with underlying health conditions.
The state has also directed retail pharmacy and municipal sites, and other vaccine partners, to hold their inoculations using the vaccine.
“Due to this information, we are going to pause on the use of the J&J until we learn more information,” Tricia Washburn, chief of the office of immunization for RIDOH, said of the recommendation by the Food and Drug Administration and the U.S. Centers for Disease Control that states pause their administration of the vaccine.
Assuming the federal review takes only a few days, the state doesn’t expect that the pause will impact its vaccine rollout. If it lasts longer, it will have to reevaluate, according to Tom McCarthy, the director of COVID response for the R.I. Department of Health.
The state is still scheduled to open eligibility for the vaccines on April 19 to all Rhode Islanders age 16 and up.
No one from Rhode Island is among the reported instances of blood clotting that sparked the federal review.
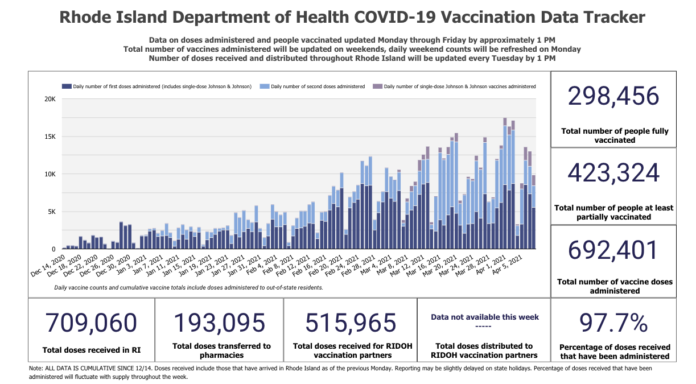
To date, about 31,000 doses of the Johnson & Johnson vaccine have been administered in Rhode Island.
The vaccine put on hold Tuesday amounts to about 3,400 doses, McCarthy said. Of those, the state has enough Moderna and Pfizer vaccine to substitute for about 2,000 Johnson & Johnson appointments. About 300 people will have to have rescheduled appointments, and they will be contacted by their retail pharmacy, he said.
The state has had a decreased supply of the one-shot, Johnson & Johnson vaccine in recent weeks due to a production error at the Baltimore manufacturing site for the vaccine. It was not immediately clear on Tuesday how many Johnson & Johnson doses were going to be held, on pause, while the federal Food and Drug Administration conducts a review of the vaccine.
According to a report in the New York Times, six recipients in the U.S. developed a rare blood clot disorder within two weeks of getting the Johnson & Johnson vaccine. All were women. One died. Almost 7 million people in the U.S. have received the vaccine.
Mary MacDonald is a staff writer for the PBN. Contact her at macdonald@pbn.com.
Updates throughout to add information from state officials.












